thiamine as antioxidant https://onlinelibrary.wiley.com/doi/pdf/10.1002/vjch.201900081
Unraveling the antioxidant potential of thiamine: Thermochemical and kinetics studies in aqueous phase using DFT
radical adduct formation at C12 (nucleophilic lower e-density site between N and S) has the most negative gibbs free energy change. 4 types of ROS-relevant reactions (HT RAF PT SET) => a paper can be written on each. ascorbic acid is broader than thamine in absorbing a broader range of hydroxyl HO and HOO reactions (with higher negative values in radical adduct formation AND FHT).
^this paper made me understand so much more than almost any paper i read before.
single electron transfer (SET), hydrogen transfer (HT), and radical adduct formation (RAF). Rate constants and branching ratios of the different channels of reaction are provided, as well as an interpretation of the UV–vis spectra. CAP is predicted to react faster in aqueous solution than in nonpolar media with oxygenated free radicals, and it was found to be a more efficient scavenger than melatonin and caffeine. It was also found that while SET does not contribute to the overall reactivity of CAP toward •OOH, •OOCH₃, and •OCH₃ radicals, it might be important for the reactions with more electrophilic radicals such as •OH, •OCCl₃, and •OOCCl₃. The main process, responsible for the peroxyl scavenging activity of CAP, was found to be the HT from the OH phenolic group. For the reaction with •OCH₃, on the other hand, the HT from allylic sites are predicted to be the main channels of reaction
also vitA by SAME authors: Is Vitamin A an Antioxidant or a Pro-oxidant? - PubMed (why do these studies all come from universities that are well-off the prestige hierarchy?)
===========
more info on RAF: Comparative Analysis of Radical Adduct Formation (RAF) Products and Antioxidant Pathways between Myricetin-3-O-Galactoside and Myricetin Aglycone . cross-relevance for ALL flavonols that can be glycosylated)
Flavonol is well known as an effective natural antioxidant. Experimental [13,14,15] and theoretical studies [16,17,18] have indicated that the antioxidant activity of flavonol is closely associated with the presence of 3-OH. Accordingly, 3-O-galactosylation is believed to reduce the antioxidant activity of flavonol, although no study on the mechanism of this reduction has been conducted.
Consequently, myricetin-3-O-galactoside (M3OGa) and its myricetin aglycone were selected as the representatives for the comparative study. As shown in Figure 1A, M3OGa bears a β-galactose residue at its 3-O position; thus, it can be regarded as the 3-O-galactosylation derivative of myricetin. If any difference in their antioxidant activities exists, it can be attributed to 3-O-galactosylation. Recently, M3OGa has been reported to coexist with its myricetin aglycone in white myrtle [11] and Nelumbo nucifera [12]. Their coexistence in the same plant has actually enhanced the comparability and biologically relevance of this comparative study.
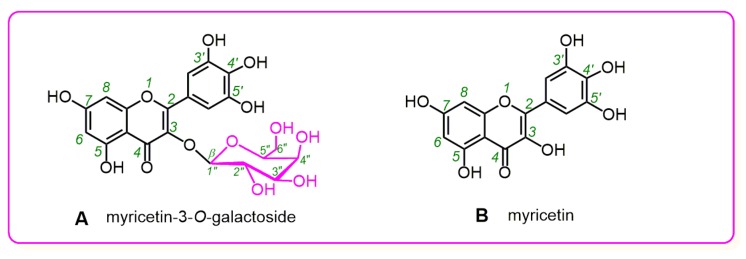
Figure 1
Structures of myricetin-3-O-galactoside (M3OGa) (A) and myricetin (B).
In the comparative study, the final products of the interaction of M3OGa and myricetin aglycone with α,α-diphenyl-β-picrylhydrazyl radical (DPPH•) were severally analyzed using leading-edge ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UPLC-ESI-Q-TOF-MS) technology to test the possibility of radical adduct formation (RAF). The high resolution of the Q-TOF-MS technology ensures the reliability of the chemical analysis. Based on the RAF product analysis, M3OGa and myricetin were further investigated for their antioxidant pathways using relevant chemical approaches. Expectedly, the series of investigative experiments will provide profound knowledge on the mechanism of the reduction of the antioxidant activity of flavonol by 3-O-galactosylation.
In addition, the understanding of the 3-O-galactosylation process is expected to be of benefit to other types of 3-O-glycosylation processes, such as 3-O-glucosylation, 3-O-rhamnglycosylation, and 3-O-arabinosylation. This is because these 3-O-glycosylation processes are essentially not different from 3-O-galactosylation, and flavonoid-3-O-glycosides are present in plants (e.g., myricetin-3-O-glucoside, myricetin-3-O-rhamnoside, and myricetin-3-O-arabinoside). From an antioxidant chemistry viewpoint, flavonol (or its glucoside) has the same antioxidant pathways as those of other phytophenols [19,20,21,22]. Thus, the analysis of the RAF products, based on the UPLC-ESI-Q-TOF-MS technology, will provide novel and reliable insights on the antioxidant chemistry of all types of phytophenols, especially flavonoid 3-O-glucosides (e.g., isorhamnetin 3-O-galactoside [7], hyperin [8], trifolin [9], and syringetin 3-O-galactoside [10]) and anthocyanin 3-O-galactosides (e.g., cyanidin-3-O-galactoside [23] and delphinidin-3-O-galactoside [24]).
the 3-OH part of the flavonol is the one that protects against RAF (3-hydroxy-2-phenylchromen-4-one ). the OH in flavonol IS the active site
more paper: Capsaicin, a Tasty Free Radical Scavenger: Mechanism of Action and Kinetics B - PubAg

