Hello, everyone! I am thrilled to introduce my recently published paper titled “Causality of Aging Hallmarks,” which is available in the journal Aging and Disease at
This paper delves into the fundamental causes of aging and explores the causal relationships between various aging hallmarks, aiming to provide a comprehensive understanding of the aging process and potential interventions.
Research Background
Aging is a complex biological process that affects all living organisms, leading to a gradual decline in physiological functions and an increased risk of age-related diseases. Traditional approaches to studying aging often focus on individual diseases or symptoms, but this paper proposes that a more effective strategy is to address the root causes of aging itself. In 2023, a review titled “Hallmarks of aging: An expanding universe” listed twelve major hallmarks of aging, but it did not provide causal arguments for each hallmark. This gap in understanding motivated my research, as interventions targeting the consequences of aging are less effective than those targeting the underlying causes.
The Fundamental Cause of Cellular Senescence
The essence of aging, according to my research, is a genetic program regulated at the DNA level. Gene expression profiles change throughout life, transitioning from embryonic development to maintenance of health and reproduction, and finally to the disruption of normal physiological functions in old age. This transition is driven by changes in telomere and ribosomal DNA (rDNA) arrays, which mediate cellular aging through the P53 pathway. The nucleus is identified as the site that determines cellular aging, with telomeres and rDNA arrays acting as “counters” that regulate gene expression and cellular function over time.
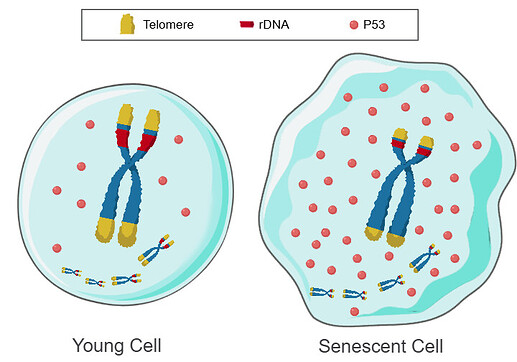
Figure 1: Telomere DNA and ribosomal DNA co-regulation model for cell senescence
Left. Chromosomes with long arrays of telomeres and rDNA: P53 is rapidly degraded, P53 levels are low, and the cell is youthful.
Right. Chromosomes with short arrays of telomeres and rDNA: P53 is slowly degraded, P53 levels are high, and the cell is aged.
Causality of the 12 Aging Hallmarks
The paper explores the causal relationships between the twelve major hallmarks of aging and P53, a master regulator of aging. These hallmarks include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, disabled macroautophagy, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis. Each hallmark is shown to be influenced by the shortening of telomeres and rDNA arrays, mediated through P53.
For example, genomic instability is linked to the downregulation of DNA methyltransferase DNMT1, which is regulated by P53. Similarly, the decline in autophagy and mitochondrial function is shown to be a consequence of aging rather than a cause, as interventions targeting these pathways have limited effects on lifespan. The accumulation of senescent cells and chronic inflammation are also shown to be downstream effects of the aging process, driven by the upregulation of P53.
Conclusions and Future Directions
The findings of this paper suggest that the shortening of telomeres and rDNA arrays is the fundamental cause of cellular senescence and aging. Interventions that target these arrays, such as lengthening telomeres and rDNA, may hold the key to reversing aging and extending lifespan. Future research should focus on developing safe and effective methods to manipulate these genetic “counters” and explore their potential for rejuvenation and longevity.
I hope this introduction gives you a glimpse into the key findings and implications of my paper. I welcome any questions or discussions on this topic, and I look forward to further exploring the fascinating field of aging and longevity with all of you on the LongevityBase Forum. Thank you!